COVID-19 Update: May 12, 2020
COVID-19 update for Tuesday, May 12 as of 3:00 p.m.
Pennsylvania Update
Department of Health
The Department of Health has issued an advisory calling on skilled nursing facilities to develop and implement plans to perform COVID-19 tests on their residents and employees.
 Department of Health Daily Briefing
Department of Health Daily Briefing
- Nearly 14,000 residents of long-term-care facilities and people who work at those facilities have now tested positive for COVID-19.
- As have nearly 4000 other health care workers.
- The state is introducing a new testing strategy for the residents and employees of skilled nursing facilities that calls for testing every resident of every such facility. All patients who are returning to such facilities from hospitals must be tested and then accommodated appropriately. The Department of Health issued guidance on its new approach today. This guidance, linked above, calls for different levels and frequency of testing for different facilities based on how many COVID-19 cases they have had and how recently they have had them.
- Secretary Levine said the state is introducing this strategy now because it is the first time it has had sufficient access to testing supplies and equipment and laboratory resources to undertake such an effort.
- She said the department also has directed skilled nursing facilities to report daily, starting May 17, on their testing activity and case counts. This information will be reported publicly.
Department of Human Services/Office of Long-Term Living
 OLTL has issued a reminder to OLTL-regulated programs operating in counties that are moving from red to yellow that previous guidance that applied to those programs remains in effect. Specifically:
OLTL has issued a reminder to OLTL-regulated programs operating in counties that are moving from red to yellow that previous guidance that applied to those programs remains in effect. Specifically:
- For programs involving home and community-based services, this DHS memo to Community HealthChoices managed care plans addressing temporary changes in the Community HealthChoices program and this notice to OBRA waiver service coordinators and providers describing temporary changes of the OBRA waiver.
- For nursing facilities, this DHS clarification guidance for pre-admission screening resident review (PASRR); Department of Health interim guidance on visitation in nursing facilities during the COVID-19 emergency; and CMS guidance for infection control and prevention.
- For Living Independence for the Elderly (LIFE) programs, OLTL’s LIFE provider guidance related to COVID-19.
- For assisted living residents and personal care homes, the Department of Health’s interim guidance on visitation to nursing facilities during the COVID-19 pandemic; CMS’s guidance for infection control and prevention; OLTL’s guidance suspending and restricting license requirements for personal care homes and assisted living residences; and DHS’s visitation restrictions in personal care homes and assisted living residences.
Federal Update
Congress
House Democrats have unveiled their newest pandemic relief bill. The overall bill is estimated to cost around $3 trillion. A few of the health care provisions included in this bill are:
 $100 billion for eligible health care providers to be distributed through the Health Care Provider Relief Fund. This is in addition to the $175 billion Congress has already appropriated for providers. This bill would include a prescriptive and structured methodology for distribution of the Health Care Provider Relief Fund that would include, among other things:
$100 billion for eligible health care providers to be distributed through the Health Care Provider Relief Fund. This is in addition to the $175 billion Congress has already appropriated for providers. This bill would include a prescriptive and structured methodology for distribution of the Health Care Provider Relief Fund that would include, among other things:- quarterly relief funds based on hospital cost reports, with aid providers already received from the relief fund subtracted from the quarterly amount
- eligibility to receive up to 60 percent of revenue lost in comparison to the previous year, to help compensate for pandemic-related expenses
- a requirement that recipients not charge COVID-19 patients and not engage in balance billing
- Reduces interests rates and lengthens the payback period for Part A and Part B advance payments.
- $75 billion for testing, contact-tracing, and other activities.
- Prohibits the administration from finalizing the Medicaid Fiscal Accountability Rule (MFAR) during the current public health emergency.
- $7.6 billion to health centers.
- $4.5 billion to NIH to expand COVID-10-related research.
- $3 billion for the Substance Abuse and Mental Health Services Administration (SAMHSA).
- Increases federal medical assistance percentage (FMAP) 14 percentage points from July 1, 2020 through June 30, 2021.
- Temporarily increases Medicaid DSH allotments to states by 2.5 percent.
- Eliminates cost-sharing for most patients for COVID-19 treatment.
These are only a few of the health care provisions included in this sprawling bill. You can find a summary of the bill here and the text of the bill here.
The House is expected to vote on the bill this Friday but the Senate is working on its own plan and is not expected to take up any new pandemic relief legislation until after Memorial Day.
Centers for Disease Control and Prevention
- The CDC has published information about factors to consider when purchasing ventilators from another country.
- The CDC has updated its FAQ on infection prevention and control in health care settings.
- The CDC has updates its interim laboratory biosafety guidelines for handling and processing specimens associated with COVID-19.
Food and Drug Administration
- The FDA has published guidance for industry and investigators addressing pre-investigational new drug application (IND) meeting requests for COVID-19-related drugs and biological products.
- The FDA has published guidance on developing drugs and biological products for the treatment or prevention of COVID-19.
- The FDA has issued an emergency use authorization for a specific commercial laboratory test to diagnose COVID-19.
 Occupational Safety and Health Administration
Occupational Safety and Health Administration
OSHA issued an alert with information about helping to keep dental industry practitioners safe during the COVID-19 emergency. See the announcement here and the alert itself here.
Resources to Consult
Pennsylvania Department of Human Services
Pennsylvania Department of Health
Centers for Disease Control and Prevention
(To receive this daily update directly, sign up for our mailing list at info@pasafetynet.org.)
 The Department of Health has posted
The Department of Health has posted  HHS has announced its
HHS has announced its  CMS COVID-19 Office Hours Calls (Tuesdays and Thursdays at 5:00 – 6:00 PM Eastern)
CMS COVID-19 Office Hours Calls (Tuesdays and Thursdays at 5:00 – 6:00 PM Eastern) The CDC has published
The CDC has published  Governor Wolf announced that
Governor Wolf announced that  The Department of State has published a notice that it will permit
The Department of State has published a notice that it will permit  The FDA has posted a letter to caregivers
The FDA has posted a letter to caregivers  Department of Human Services
Department of Human Services HHS’s Health Resources and Services Administration, which is administering the program through which providers can be reimbursed for COVID-19-related services they provide to uninsured patients, has
HHS’s Health Resources and Services Administration, which is administering the program through which providers can be reimbursed for COVID-19-related services they provide to uninsured patients, has  The CDC has posted
The CDC has posted  With 825 new cases yesterday, Pennsylvania has surpassed 50,000 people diagnosed with COVID-19. 2500 of them have passed away.
With 825 new cases yesterday, Pennsylvania has surpassed 50,000 people diagnosed with COVID-19. 2500 of them have passed away. The Department of Labor has issued additional guidance about 100 percent federal reimbursement of certain state short-term compensation payments and other changes in short-term compensation programs. The guidance seeks to address “…how states can take advantage of this program as they look to re-open their businesses.” For further information see the department’s
The Department of Labor has issued additional guidance about 100 percent federal reimbursement of certain state short-term compensation payments and other changes in short-term compensation programs. The guidance seeks to address “…how states can take advantage of this program as they look to re-open their businesses.” For further information see the department’s 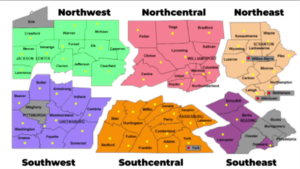 Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties.
Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties. MACPAC has written to CMS administrator Seema Verma to express its concern that
MACPAC has written to CMS administrator Seema Verma to express its concern that  Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE.
Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE. Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE.
Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE. Pennsylvania Senate Democrats
Pennsylvania Senate Democrats  The FDA has posted
The FDA has posted