Number of Uninsured Children Grows in PA
The number of uninsured children in Pennsylvania grew, but just slightly, between 2016 and 2019.
 That’s according to a new study from the Center for Children and Families at Georgetown University’s Health Policy Institute.
That’s according to a new study from the Center for Children and Families at Georgetown University’s Health Policy Institute.
According to the study, the uninsured rate among children in Pennsylvania rose from 4.4 percent in 2016 to 4.6 percent in 2019 while the number of uninsured children rose from 126,000 to 128,000 during that same period of time.
Learn more about the changes in Pennsylvania, national trends, and why these numbers have grown in the Georgetown study “Children’s Uninsured Rate Rises by Largest Annual Jump in More Than a Decade.”
 The enrollment increase can be traced to rising unemployment, with many people losing their employer-sponsored health insurance. The new figures cover five months, from February through June, the latter four of which marked the beginning of the COVID-19 pandemic.
The enrollment increase can be traced to rising unemployment, with many people losing their employer-sponsored health insurance. The new figures cover five months, from February through June, the latter four of which marked the beginning of the COVID-19 pandemic. The Department of State has
The Department of State has  This week’s
This week’s  Provider Relief Fund
Provider Relief Fund CMS has posted the document “
CMS has posted the document “ The CDC has updated its
The CDC has updated its 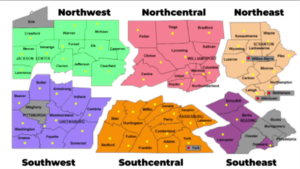 Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties.
Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties. Yesterday CMS released a second interim final rule with comment period announcing a new round of regulatory waivers and rule changes to provide additional flexibility to the health care system as the COVID-19 crisis continues and the country begins to reopen.
Yesterday CMS released a second interim final rule with comment period announcing a new round of regulatory waivers and rule changes to provide additional flexibility to the health care system as the COVID-19 crisis continues and the country begins to reopen. MACPAC has written to CMS administrator Seema Verma to express its concern that
MACPAC has written to CMS administrator Seema Verma to express its concern that  Department of State
Department of State CMS has posted a
CMS has posted a  The DEA has published a letter that grants to the satellite hospitals and clinics of DEA-registered hospitals, under certain conditions, the
The DEA has published a letter that grants to the satellite hospitals and clinics of DEA-registered hospitals, under certain conditions, the  Governor Wolf/Financial Assistance for Hospitals
Governor Wolf/Financial Assistance for Hospitals Department of Human Services
Department of Human Services The FDA has issued guidance on best
The FDA has issued guidance on best  Governor Wolf recommended that all Pennsylvanians wear masks outside the home. He elaborates on this recommendation in a
Governor Wolf recommended that all Pennsylvanians wear masks outside the home. He elaborates on this recommendation in a  The CDC has issued new
The CDC has issued new  HHS’s Office of Civil Rights has announced that it will not impose penalties for violations of certain provisions of the HIPAA privacy rule against health care providers or their business associates for the good faith uses and disclosures of protected health information by business associates for public health and health oversight activities during the COVID-19 nationwide public health emergency. See the Office of Civil Rights
HHS’s Office of Civil Rights has announced that it will not impose penalties for violations of certain provisions of the HIPAA privacy rule against health care providers or their business associates for the good faith uses and disclosures of protected health information by business associates for public health and health oversight activities during the COVID-19 nationwide public health emergency. See the Office of Civil Rights  The number of new COVID-19 cases yesterday rose 26 percent over the previous day.
The number of new COVID-19 cases yesterday rose 26 percent over the previous day. Pennsylvania tax revenues were $294.6 million short of official estimates in March, or 6.2 percent, according
Pennsylvania tax revenues were $294.6 million short of official estimates in March, or 6.2 percent, according  The Central Penn Business Journal has published an interview with Pennsylvania Department of Health Secretary Rachel Levine, who is leading the state’s response to the COVID-19 pandemic. Read that interview
The Central Penn Business Journal has published an interview with Pennsylvania Department of Health Secretary Rachel Levine, who is leading the state’s response to the COVID-19 pandemic. Read that interview