COVID-19 Update: Thursday, October 1
The following is the latest COVID-19 information from the state and federal governments as of 3:30 p.m. on Thursday, October 1.
Pennsylvania Update
Governor Wolf
The Wolf administration has introduced an enhanced strategy to make COVID-19 testing more accessible, available, and adaptable as the state learns more about the virus. The test is built around four tiers for determining priority for testing. Learn more about the strategy here.
Department of Health
 The Department of Health has published an updated FAQ with interim guidance for skilled nursing facilities that addresses questions related to recent department guidance during the COVID-19 emergency.
The Department of Health has published an updated FAQ with interim guidance for skilled nursing facilities that addresses questions related to recent department guidance during the COVID-19 emergency.- The Department of Health has updated its on-site survey guidance for hospitals and ambulatory surgical facilities to add instructions to notify the department if a surveyor comes in close contact with a confirmed or probable COVID-19 case in the course of a survey.
- The Department of Health addressed questions from skilled nursing facilities about respirator fit testing and home visitation guidance in its weekly COVID-19 Guidance Wrap-up document.
Department of Health – by the numbers
- This week’s joint news release from Governor Wolf and the Department of Health revealed that during the week of September 18 to September 24 the number of new COVID-19 cases in Pennsylvania fell 8.7 percent.
- The total number of COVID-19 deaths in Pennsylvania since the start of the pandemic climbed today past 8100.
- Centre and Northumberland counties are experiencing the highest rate of community transmission.
- For the past week the number of daily cases has risen and has included two of the highest new case counts in a month, including today’s new case count, the highest since September 3.
- The continued high numbers are driven in part by persistent increases in the number of young people between the ages of 19 and 24 who are contracting COVID-19.
- The number of Pennsylvanians currently hospitalized with COVID-19 has risen in nine of the past ten days. The number of such patients breathing with the help of a ventilator, on the other hand, has changed little in the past week.
- More than 10,800 health care workers in the state have contracted COVID-19.
- 20 percent of the beds in Pennsylvania’s acute-care hospitals are currently unoccupied, as are 25 percent of adult ICU beds, 16 percent of pediatric ICU beds, 38 percent of pediatric beds, and 40 percent of airborne isolation rooms.
Department of Human Services
 DHS has issued a Medical Assistance Bulletin on COVID-19 specimen collection and testing at pharmacies. It takes effect immediately.
DHS has issued a Medical Assistance Bulletin on COVID-19 specimen collection and testing at pharmacies. It takes effect immediately.
DHS’s Office of Developmental Programs has posted updated guidance to Individual Support Planning Teams on the criteria for requesting a cap exception for the Person/Family Directed Support (P/FDS) and Community Living Waivers.
Federal Update
 Provider Relief Fund
Provider Relief Fund
- HHS announced the planned distribution of $20 billion in new funding for providers on the front lines of the COVID-19 pandemic. Under this Phase 3 General Distribution allocation, providers that have already received Provider Relief Fund payments will be invited to apply for additional funding that considers financial losses and increased expenses experienced due to COVID-19. Previously ineligible providers, such as those that began practicing in 2020, will also be invited to apply, and an expanded group of behavioral health providers confronting the emergence of increased mental health and substance use issues exacerbated by the pandemic will also be eligible for relief payments.
- This new distribution should be especially helpful for providers that have received minimum or no targeted relief, such as safety-net, high-impact, or rural distributions.
- Providers can begin applying for funds on Monday, October 5, 2020 and the application deadline is November 6.
- According to HHS’s news release,
- All provider submissions will be reviewed to confirm they have received a Provider Relief Fund payment equal to approximately 2 percent of patient care revenue from prior general distributions. Applicants that have not yet received Relief Fund payments of 2 percent of patient revenue will receive a payment that, when combined with prior payments (if any), equals 2 percent of patient care revenue.
- With the remaining balance of the $20 billion budget, HRSA will then calculate an equitable add-on payment that considers the following:
- A provider’s change in operating revenues from patient care.
- A provider’s change in operating expenses from patient care, including expenses incurred related to coronavirus.
- Payments already received through prior Provider Relief Fund distributions.
Go here to learn more about the distribution.
- HHS has published a new summary of reporting requirements for recipients of the Provider Relief Fund grants.
Department of Health and Human Services
Centers for Medicare & Medicaid Services
 Congress has passed, and the president has now signed, a continuing resolution to fund the federal government through December 11. The resolution includes a provision that would change the terms under which providers must repay federal CARES Act money they received through the Medicare Accelerated and Advance Payment program, which is administered by CMS. Now, recoupment will begin only a year after providers received their loan and recoupment is reduced from 100 percent to 25 percent during the first 11 months of repayment and 50 percent for the six following months, with hospitals now having 29 months to repay their loans in full before they would need to begin paying interest. That interest rate, too, is lowered under the continuing resolution from 9.6 percent to 4.0 percent.
Congress has passed, and the president has now signed, a continuing resolution to fund the federal government through December 11. The resolution includes a provision that would change the terms under which providers must repay federal CARES Act money they received through the Medicare Accelerated and Advance Payment program, which is administered by CMS. Now, recoupment will begin only a year after providers received their loan and recoupment is reduced from 100 percent to 25 percent during the first 11 months of repayment and 50 percent for the six following months, with hospitals now having 29 months to repay their loans in full before they would need to begin paying interest. That interest rate, too, is lowered under the continuing resolution from 9.6 percent to 4.0 percent.- CMS has updated its compendium of temporary waivers and flexibilities for teaching hospitals, teaching physicians, and medical residents during the COVID-19 pandemic. A new flexibility, on page 2 of the document, explains that instead of requiring that new Medicare GME affiliation agreements be submitted to CMS and MACs by July 1, 2020 for the academic year starting July 1, 2020 and amendments to Medicare GME affiliation agreements be submitted to CMS and the MACs by June 30, 2020 for the academic year ending June 30, 2020, CMS is permitting hospitals to submit new and/or amended Medicare GME affiliation agreements as applicable to CMS and the MACs by January 1, 2021.
- CMS has updated its COVID-19 testing methodology for nursing homes by revising the methodology it employs to determine the rate of COVID-19 positivity in counties across the country.
- CMS has published guidance addressing the emergency preparedness testing exercise requirements for COVID-19. CMS regulations for emergency preparedness require specific testing exercises to validate facilities’ emergency programs.
Food and Drug Administration
- The FDA has published state-by-state and national forecasts of COVID-19 hospitalizations for the next four weeks.
- The FDA has issued emergency use authorization for a prediction system to be used by health care providers in hospitals for adult patients with confirmed COVID-19 for the computation of proprietary patient status indices referred to as Respiratory Decompensation Status and Hemodynamic Instability Status (collectively referred to as “risk level determinations”) as an adjunct to patient monitoring during the COVID-19 outbreak.
National Institutes of Health
 The NIH has awarded $234 million to improve COVID-19 testing for underserved and vulnerable populations. The program will support 32 institutions and focus on populations disproportionately affected by the pandemic, including African Americans, American Indians/Alaskan Natives, Latinos/Latinas, Native Hawaiians, older adults, pregnant women, and those who are homeless or incarcerated.
The NIH has awarded $234 million to improve COVID-19 testing for underserved and vulnerable populations. The program will support 32 institutions and focus on populations disproportionately affected by the pandemic, including African Americans, American Indians/Alaskan Natives, Latinos/Latinas, Native Hawaiians, older adults, pregnant women, and those who are homeless or incarcerated.
Federal Communications Commission
The FCC has extended from September 30 to December 31, 2020 the deadline for recipients of funding from its $200 million COVID-19 Telehealth Program to purchase eligible devices and implement eligible services.
Resources to Consult
Pennsylvania Department of Human Services
Pennsylvania Department of Health
Centers for Disease Control and Prevention
 Governor Wolf and Health Secretary Levine introduced “COVID-19 Alert PA,” a new cell phone app that the state hopes will facilitate contact tracing. The app, which works on Android and Apple cell phones, uses bluetooth technology to identify people who were recently near someone who has been diagnosed with COVID-19. The app notifies individuals who may have been exposed and directs them to resources but does not provide the name of the person who was diagnosed and does not provide the state with the names of people who may have been exposed, protecting the privacy of everyone involved and leaving responsibility to act at the discretion of the people with the app on their phone. The app, which is free, does not track people and their location. Learn more from this
Governor Wolf and Health Secretary Levine introduced “COVID-19 Alert PA,” a new cell phone app that the state hopes will facilitate contact tracing. The app, which works on Android and Apple cell phones, uses bluetooth technology to identify people who were recently near someone who has been diagnosed with COVID-19. The app notifies individuals who may have been exposed and directs them to resources but does not provide the name of the person who was diagnosed and does not provide the state with the names of people who may have been exposed, protecting the privacy of everyone involved and leaving responsibility to act at the discretion of the people with the app on their phone. The app, which is free, does not track people and their location. Learn more from this  HHS has
HHS has  The Courts
The Courts According to the study,
According to the study, The Department of State has
The Department of State has  Provider Relief Fund
Provider Relief Fund CMS has posted the document “
CMS has posted the document “ The CDC has updated its
The CDC has updated its  Independent Fiscal Office
Independent Fiscal Office Governor Wolf has signed a second renewal of his 90-day disaster declaration for the COVID-19 pandemic. This declaration provides for increased support for state agencies involved in the continued response to the virus and recovery during reopening, including expediting supply procurement and lifting certain regulations to allow for efficient and effective mitigation. The disaster declaration also has facilitated waivers and extensions to support Pennsylvanians, Pennsylvania businesses, and Pennsylvania caregivers during the pandemic. Learn more from
Governor Wolf has signed a second renewal of his 90-day disaster declaration for the COVID-19 pandemic. This declaration provides for increased support for state agencies involved in the continued response to the virus and recovery during reopening, including expediting supply procurement and lifting certain regulations to allow for efficient and effective mitigation. The disaster declaration also has facilitated waivers and extensions to support Pennsylvanians, Pennsylvania businesses, and Pennsylvania caregivers during the pandemic. Learn more from 
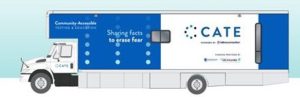 The Department of Health has unveiled “CATE” (Community-Accessible Testing and Education”), a recreational vehicle that has been equipped as a mobile COVID-19 testing and education unit that will travel the state offering free COVID-19 tests and education in medically underserved communities in 16 counties. Established and operated by the organization Latino Connection, staffed by the Welsh Mountain Health Centers, and funded in part by the state, Highmark, and Independence Blue Cross, CATE has more than 30 stops scheduled during September, the first half of them in the Philadelphia area and then moving westward across the state. Appointments are not needed and CATE’s tests will be performed by the state’s lab in Exton, which is producing results in 24 to 48 hours. Learn more about CATE, its origins, and its scheduled stops in this Department of Health
The Department of Health has unveiled “CATE” (Community-Accessible Testing and Education”), a recreational vehicle that has been equipped as a mobile COVID-19 testing and education unit that will travel the state offering free COVID-19 tests and education in medically underserved communities in 16 counties. Established and operated by the organization Latino Connection, staffed by the Welsh Mountain Health Centers, and funded in part by the state, Highmark, and Independence Blue Cross, CATE has more than 30 stops scheduled during September, the first half of them in the Philadelphia area and then moving westward across the state. Appointments are not needed and CATE’s tests will be performed by the state’s lab in Exton, which is producing results in 24 to 48 hours. Learn more about CATE, its origins, and its scheduled stops in this Department of Health 

