COVID-19 Update: May 1, 2020
COVID-19 update for Friday, May 1 as of 4:00 p.m.
Pennsylvania Update
Reopening Pennsylvania
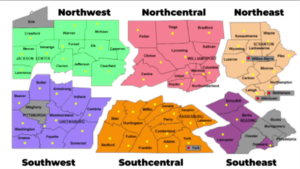 Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties.
Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties.
Health Secretary Levine encouraged residents of the 24 counties to continue the recent practices of social distancing and use of face masks. For more information on the reopening, what it means, and how it will work, see this news release from the governor’s office. Additional resources of interest include:
- the risk-based decision support tool developed by Carnegie Mellon University that was used to help guide decision-making on which regions and counties to reopen;
- a state document on contact tracing;
- a state document on COVID-19 testing; and
- the standards the state is employing when making reopening decisions.
 Department of Health
Department of Health
The Department of Health has updated its interim guidance for collecting clinical specimens for COVID-19 testing.
Department of Health Daily Briefing
- Governor Wolf and Secretary Levine discussed the reopening of 24 counties during the daily briefing.
- The governor said that southwestern and south-central Pennsylvania look like good candidates for reopening in the near future.
- Secretary Levine discussed how contact tracing would be undertaken in the 24 counties, listing the resources that would be involved and holding out the possibility that the state may hire additional people to help, if needed.
- Community-based testing will be available in the 24 counties for people with COVID-19 symptoms.
- The governor said the state has already reduced spending in the current fiscal year in light of reduced revenue and that he will be working with the legislature on an FY 2021 budget with the understanding that revenues will continue to be down from past levels.
- 2677 people are currently hospitalized with COVID-19 and 561 of them have required help from a ventilator.
- 40 percent of the state’s acute-care beds and 47 percent of its ICU beds are currently unoccupied and 70 percent of ventilators are now idle.
Department of Human Services
DHS has posted information about all-patient refined-diagnosis related groups (APR-DRGs) to be updated with COVID-19 billing codes.
Department of Revenue
The Revenue Department announced that Pennsylvania collected $2.2 billion in General Fund revenue in April, which was $2.2 billion, or 49.7 percent, less than anticipated. For the fiscal year-to-date General Fund collections total $27.5 billion, which is $2.2 billion, or 7.4 percent, below estimate. It is too soon to draw any conclusions about how this will affect negotiations for the state’s FY 2021 budget and its Medicaid program but this loss of revenue clearly poses a challenge state officials will need to address.
Federal Update
Follow-Up to Thursday’s Interim Final Rule Release
 Yesterday CMS released a second interim final rule with comment period announcing a new round of regulatory waivers and rule changes to provide additional flexibility to the health care system as the COVID-19 crisis continues and the country begins to reopen.
Yesterday CMS released a second interim final rule with comment period announcing a new round of regulatory waivers and rule changes to provide additional flexibility to the health care system as the COVID-19 crisis continues and the country begins to reopen.
These changes included some of the telehealth flexibilities providers have asked for in recent weeks, such as hospital reimbursement for the originating site when telehealth services for Medicare patients are furnished by a physician or practitioner who ordinarily practices in a hospital outpatient department to a patient who is located at home or other applicable temporary expansion location that has been made provider-based to the hospital; an expanded list of telehealth codes that may be offered with audio-only technology; and an expanded list of professionals who may bill for telehealth visits. CMS has also increased reimbursement for three of the audio-only evaluation and management telehealth services described with codes 99441-99443.
Teaching hospitals are permitted to expand their number of beds without affecting indirect medical education (IME) bed ratios; to count the time of residents sent to other hospitals; and to permit teaching physicians to remotely review services provided by residents immediately after a patient visit.
The interim final rule implements sweeping changes for many provider types. CMS has updated its provider-specific fact sheets to include this new information. Please let us know if you have any questions that are not answered in the fact sheets.
- Home Health Agencies
- Physicians and Other Practitioners
- Ambulances
- Hospitals
- Teaching Hospitals, Teaching Physicians and Medical Residents
- Long Term Care Facilities (Skilled Nursing Facilities and/or Nursing Facilities)
- Hospices
- Inpatient Rehabilitation Facilities
- Long Term Care Hospitals & Extended Neoplastic Disease Care Hospitals
- Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)
- Laboratories
- End Stage Renal Disease (ESRD) Facilities
- Durable Medical Equipment
- Participants in the Medicare Diabetes Prevention Program
- Medicare Advantage and Part D Plans
- State Medicaid & Basic Health Programs
- Medicare Shared Savings Program Participants
Centers for Medicare & Medicaid Services
- The White House announced that CMS will be distributing $12 billion of CARES Act provider relief to 395 hospitals that have cared for 70 percent of the diagnosed COVID-19 cases. The majority of the funds will go to providers in New York, New Jersey, and Illinois. $2 billion of this money will go toward a Medicare DSH adjustment for those hospitals. We are still awaiting additional details.
- CMS has announced the creation of an independent commission to conduct a comprehensive assessment of the nursing home response to the COVID-19 emergency. See CMS’s news release and a fact sheet.
- CMS has updated it EMTALA FAQ for hospitals to address COVID-19-related issues.
Department of Health and Human Services
- HHS has launched a new COVID-19 Uninsured Program Portal, which will be the vehicle through which providers seek reimbursement for the COVID-19-related services they provide to uninsured patients. HHS has now released a companion billing guide with more detailed information about the process for electronic data interchange (EDI) claims submission. Providers are encouraged to sign up as quickly as possible to avoid processing delays.
- HHS’s Office of the Inspector General has updated its FAQ on how it is exercising its discretion in the enforcement of requirements directly connected to the COVID-19 emergency. The FAQ also describes how stakeholders can submit questions about how the OIG is applying its discretionary authority during the pandemic.
Food and Drug Administration
- The FDA has updated its list of authorized ventilators, ventilator tubing connectors, and ventilator accessories.
- The FDA has issued emergency use authorization for two new COVID-19 detection tests (here and here) and one new COVID-19 serology test.
Department of Labor
- Along with the Treasury Department, the Department of Labor has scheduled for publication in the Federal Register a notice extending certain time frames for COBRA participation during the COVID-19 crisis.
Federal Communications Commission
- The FCC has awarded 13 more grants worth $4.2 million as part of its COVID-19 Telehealth Program for health care providers to provide telehealth services during the COVID-19 emergency.
Medicaid and CHIP Payment and Access Commission
 MACPAC has written to CMS administrator Seema Verma to express its concern that the manner in which her agency is distributing CARES Act funds designated for health care providers is not directing enough of that money to safety-net hospitals and others that care for especially large numbers of Medicaid beneficiaries.
MACPAC has written to CMS administrator Seema Verma to express its concern that the manner in which her agency is distributing CARES Act funds designated for health care providers is not directing enough of that money to safety-net hospitals and others that care for especially large numbers of Medicaid beneficiaries.
Resources to Consult
Pennsylvania Department of Human Services
Pennsylvania Department of Health
Centers for Disease Control and Prevention
(To receive this daily update directly, sign up for our mailing list at info@pasafetynet.org.)
 Earlier this month the state launched a $450 million Hospital Emergency Loan Program to provide short-term loans to hospitals struggling financially with their response to the COVID-19 emergency. Today the
Earlier this month the state launched a $450 million Hospital Emergency Loan Program to provide short-term loans to hospitals struggling financially with their response to the COVID-19 emergency. Today the  Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE.
Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE. Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE.
Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE. Pennsylvania Senate Democrats
Pennsylvania Senate Democrats  The FDA has posted
The FDA has posted  DHS has sent a memo to the HealthChoices physical health managed care organizations
DHS has sent a memo to the HealthChoices physical health managed care organizations  The Trump administration has released two new documents to support its plan for reopening the country and its economy:
The Trump administration has released two new documents to support its plan for reopening the country and its economy:  The Department of Health has issued
The Department of Health has issued  Last Friday HHS deposited its second tranche of CARES Act funding in hospitals’ bank accounts. We have contacted HHS to inquire about the specifics of the funding formula, whether there are additional future attestation requirements, the timing for the next round of payments, and more.
Last Friday HHS deposited its second tranche of CARES Act funding in hospitals’ bank accounts. We have contacted HHS to inquire about the specifics of the funding formula, whether there are additional future attestation requirements, the timing for the next round of payments, and more. The Pennsylvania Health Law Center has launched a “
The Pennsylvania Health Law Center has launched a “
 The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has shared its
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has shared its  On Wednesday night Governor Wolf addressed the state about his plan to gradually reopen Pennsylvania and its economy. While the governor had previously announced some reopenings, he outlined general parameters for authorizing additional steps and further business activity.
On Wednesday night Governor Wolf addressed the state about his plan to gradually reopen Pennsylvania and its economy. While the governor had previously announced some reopenings, he outlined general parameters for authorizing additional steps and further business activity. The CDC has published information about
The CDC has published information about  Today SNAP wrote to members of Pennsylvania’s congressional delegation to ask them to support new COVID-19 and economic relief legislation that was expected to include $75 billion for hospitals. See SNAP’s letter
Today SNAP wrote to members of Pennsylvania’s congressional delegation to ask them to support new COVID-19 and economic relief legislation that was expected to include $75 billion for hospitals. See SNAP’s letter  The Department of Health new daily case counts are now the sum of two figures: “confirmed” cases that have been determined by testing plus cases that have been ruled “probable” because of an individual’s symptoms and recent contact with someone who has a confirmed case of COVID-19.
The Department of Health new daily case counts are now the sum of two figures: “confirmed” cases that have been determined by testing plus cases that have been ruled “probable” because of an individual’s symptoms and recent contact with someone who has a confirmed case of COVID-19. Congressional leaders and the Trump administration have agreed to provide $75 billion for hospitals as part of a $484 billion COVID-19 and economic relief package. This $75 billion would be addition to the money from the CARES Act and would have the same parameters as the CARES Act money.
Congressional leaders and the Trump administration have agreed to provide $75 billion for hospitals as part of a $484 billion COVID-19 and economic relief package. This $75 billion would be addition to the money from the CARES Act and would have the same parameters as the CARES Act money. Governor Wolf today announced that he was extending his stay-at-home order through May 8, at which time the state may, depending on the status of spread of COVID-19, begin permitting some industries and businesses to resume operations while still observing social distancing guidelines. Pennsylvania’s liquor stores have begun curbside pick-up and online auto sales will be permitted to resume, with notaries doing their work online. Construction projects would be permitted to resume on May 8. The administration is exploring permitting some retailers to engage in curbside pick-ups but the governor acknowledged that this presented different challenges in different places. He said the reopening of the state’s economy would be regional rather than state-wide, that some things that may be realistic in Cameron County may not be feasible in Philadelphia, and that all reopening efforts would be contingent on the progress of the COVID-19 pandemic between now and May 8. He did not speak about anything involving health care other than to note that social distancing appears to have been effective in preventing the health care system from becoming overwhelmed at the height of the crisis.
Governor Wolf today announced that he was extending his stay-at-home order through May 8, at which time the state may, depending on the status of spread of COVID-19, begin permitting some industries and businesses to resume operations while still observing social distancing guidelines. Pennsylvania’s liquor stores have begun curbside pick-up and online auto sales will be permitted to resume, with notaries doing their work online. Construction projects would be permitted to resume on May 8. The administration is exploring permitting some retailers to engage in curbside pick-ups but the governor acknowledged that this presented different challenges in different places. He said the reopening of the state’s economy would be regional rather than state-wide, that some things that may be realistic in Cameron County may not be feasible in Philadelphia, and that all reopening efforts would be contingent on the progress of the COVID-19 pandemic between now and May 8. He did not speak about anything involving health care other than to note that social distancing appears to have been effective in preventing the health care system from becoming overwhelmed at the height of the crisis. FEMA has published a
FEMA has published a