COVID-19 Update: May 7, 2020
COVID-19 update for Thursday, May 7 as of 2:30 p.m.
Pennsylvania Update
General Assembly
The House canceled its plan to meet next week, on May 11, 12, and 13, and has added May 18, 19, 20, 26, and 27 to its legislative calendar. The Senate is scheduled to return to session on May 18.
 Department of Human Services
Department of Human Services
- DHS has posted a notice rescinding prior guidance on elective services. This has been done to reflect the phased reopening of Pennsylvania and its health care system and updated guidance for hospitals and guidance for ambulatory surgical facilities from the Department of Health based on that reopening.
- DHS has sent a similar message to the managed care organizations that participate in the state’s HealthChoices physical health program.
- DHS has sent a memo to the managed care organizations that participate in the state’s Children’s Health Insurance Program (CHIP) informing them that unemployment income must be calculated for a 39-week period when determining eligibility for CHIP for the duration of the COVID-19 emergency.
Governor Wolf
Governor Wolf has signed an executive order giving health care practitioners protection against liability for good faith actions taken in response to the call to supplement the health care provider workforce during the COVID-19 pandemic. HAP and the PA Chamber of Commerce and Industry are calling for the protection to be expanded to institutional health care providers.
Governor Wolf has signed an executive order protecting businesses and individuals whose livelihood has been disrupted by COVID-19 from foreclosures or evictions through July 10.
The governor has announced the formation of a volunteer think tank to “…strategize how to heal the trauma that all Pennsylvanians are experiencing due to the COVID-19 pandemic.” This marks an expansion of the group’s original scope of work, introduced last July, which was to advise the state government on policies that are sensitive to the needs of individuals who have suffered traumatic experiences.
Department of Health
The Department of Health has posted an FAQ for families of nursing care facility staff and residents.
Department of Health Daily Briefing
 As part of its continuing reconciliation of data, the Department of Health added more than 300 people to its total of deaths from COVID-19.
As part of its continuing reconciliation of data, the Department of Health added more than 300 people to its total of deaths from COVID-19.- Among Pennsylvanians who have tested positive from COVID-19, 3437 are health care workers (including nursing home staff), 2107 are food industry workers, and more than 10,000 reside in 514 long-term-care facilities.
- 2484 Pennsylvanians are currently hospitalized for COVID-19 and 528 are on ventilators.
- 45 percent of the state’s acute-care beds and 39 percent of its ICU beds are currently unoccupied and 73 percent of its ventilators are idle.
- Governor Wolf said the state will announce more county/regional openings tomorrow.
- The state is looking at various Bluetooth technologies to facilitate contact tracing and is now working on a contract for such technology.
Federal Update
Department of Health and Human Services Provider Relief Fund
 HHS has extended the deadline for attestation and acceptance of terms and conditions for payments from the Provider Relief Fund from the current 30 days to 45 days.
HHS has extended the deadline for attestation and acceptance of terms and conditions for payments from the Provider Relief Fund from the current 30 days to 45 days.- HRSA, which is administering the program through which providers can submit claims for COVID-19-related care they provide to uninsured patients, has posted an FAQ based on a webinar it held last week to address how providers can submit those claims.
- HRSA also has posted a video demonstrating how to add and attest to the patient roster on the portal for submitting claims for COVID-19 care for the uninsured.
Department of Health and Human Services
HHS’s Office of the Assistant Secretary for Preparedness and Response’s Technical Resources Assistance Center and Information Exchange will hold a webinar titled “COVID-19: Healthcare System Operations Strategies and Experiences” next Monday, May 11 at 2:00 p.m. (eastern) to help health systems incorporate experiences and lessons learned from hospitals in New York, Louisiana, and Arizona that have been hit especially hard by COVID-19. Find information about the webinar here.
Health Resources and Services Administration
HRSA has awarded nearly $583 million to 1385 HRSA-funded health centers in all 50 states. The primary purpose of this funding is to expand COVID-19 testing. See HRSA’s announcement here and an interactive map showing award recipients here.
Centers for Medicare & Medicaid Services
 CMS has issued an interim final rule updating requirements for notification of confirmed and suspected COVID-19 cases among residents and staff in nursing homes. In the guidance, nursing homes are directed to report COVID-19 data to the CDC and the residents of nursing homes and their families and representatives.
CMS has issued an interim final rule updating requirements for notification of confirmed and suspected COVID-19 cases among residents and staff in nursing homes. In the guidance, nursing homes are directed to report COVID-19 data to the CDC and the residents of nursing homes and their families and representatives.- CMS has posted an updated list of lab test codes for COVID-19, influenza, and respiratory syncytial virus (RSV).
Centers for Disease Control and Prevention
- The CDC has updated its FAQ for infection prevention and control in health care settings.
- The CDC has created a web page to share information about its own COVID-19 serology test and its possible future uses.
- The CDC has posted guidance for cleaning and disinfecting public spaces, workplaces, businesses, schools, and homes.
- The CDC also has published separate reopening guidance for cleaning and disinfecting public spaces, workplaces, businesses, schools, and homes.
Resources to Consult
Pennsylvania Department of Human Services
Pennsylvania Department of Health
Centers for Disease Control and Prevention
(To receive this daily update directly, sign up for our mailing list at info@pasafetynet.org.)
 The Wolf administration announced that it is establishing a facility in Delaware County that has the capacity to decontaminate “tens of thousands” of N95 respirators a day. The equipment has been provided by the U.S. Department of Health and Human Services, the service is free, and among those eligible to use this service are health care facilities, first responders, and others, such as hospitals, urgent care centers, nursing homes, rehabilitation facilities, cancer centers, pharmacies, dialysis centers, assisted living facilities, clinical laboratories, emergency medical services, private practice/outpatient facilities, first responder organizations, and others. Interested organizations must register and information will be available through the Pennsylvania Department of Health, the Pennsylvania Emergency Management Agency, the Hospital and Healthsystem Association of Pennsylvania, and others. Read the Wolf administration’s announcement
The Wolf administration announced that it is establishing a facility in Delaware County that has the capacity to decontaminate “tens of thousands” of N95 respirators a day. The equipment has been provided by the U.S. Department of Health and Human Services, the service is free, and among those eligible to use this service are health care facilities, first responders, and others, such as hospitals, urgent care centers, nursing homes, rehabilitation facilities, cancer centers, pharmacies, dialysis centers, assisted living facilities, clinical laboratories, emergency medical services, private practice/outpatient facilities, first responder organizations, and others. Interested organizations must register and information will be available through the Pennsylvania Department of Health, the Pennsylvania Emergency Management Agency, the Hospital and Healthsystem Association of Pennsylvania, and others. Read the Wolf administration’s announcement  HHS’s Health Resources and Services Administration, which is administering the program through which providers can be reimbursed for COVID-19-related services they provide to uninsured patients, has
HHS’s Health Resources and Services Administration, which is administering the program through which providers can be reimbursed for COVID-19-related services they provide to uninsured patients, has  The FDA has
The FDA has  The CDC has posted
The CDC has posted  With 825 new cases yesterday, Pennsylvania has surpassed 50,000 people diagnosed with COVID-19. 2500 of them have passed away.
With 825 new cases yesterday, Pennsylvania has surpassed 50,000 people diagnosed with COVID-19. 2500 of them have passed away. The Department of Labor has issued additional guidance about 100 percent federal reimbursement of certain state short-term compensation payments and other changes in short-term compensation programs. The guidance seeks to address “…how states can take advantage of this program as they look to re-open their businesses.” For further information see the department’s
The Department of Labor has issued additional guidance about 100 percent federal reimbursement of certain state short-term compensation payments and other changes in short-term compensation programs. The guidance seeks to address “…how states can take advantage of this program as they look to re-open their businesses.” For further information see the department’s 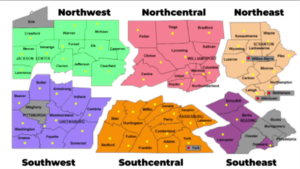 Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties.
Governor Wolf on Friday announced the reopening of 24 Pennsylvania counties in the northwestern and north-central parts of the state, moving them from red to yellow in his three-color plan for reopening the state. The governor outlined the criteria used to reach these decisions and the provisions for contact tracing and additional testing that make reopening possible. The reopening calls for reduced use but not elimination of social distancing and includes guidance on work, congregate, and social activities that are now permitted as well as those that remain restricted or prohibited. On Monday the administration will release guidance for businesses permitted to reopen in the selected counties. MACPAC has written to CMS administrator Seema Verma to express its concern that
MACPAC has written to CMS administrator Seema Verma to express its concern that  Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE.
Quality Assurance and Performance Improvement (QAPI). (New since 4/21 Release) CMS is modifying certain requirements in 42 CFR §483.75, which requires long-term care facilities to develop, implement, evaluate, and maintain an effective, comprehensive, data-driven QAPI program. Specifically, CMS is modifying §483.75(b)–(d) and (e)(3) to the extent necessary to narrow the scope of the QAPI program to focus on adverse events and infection control. This will help ensure facilities focus on aspects of care delivery most closely associated with COVID-19 during the PHE. Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE.
Training and Assessment of Aides: (New since 4/21 Release) CMS is waiving the requirement at 42 CFR §418.76(h)(2) for Hospice and 42 CFR §484.80(h)(1)(iii) for HHAs, which require a registered nurse, or in the case of an HHA a registered nurse or other appropriate skilled professional (physical therapist/occupational therapist, speech language pathologist) to make an annual onsite supervisory visit (direct observation) for each aide that provides services on behalf of the agency. In accordance with section 1135(b)(5) of the Act, we are postponing completion of these visits. All postponed onsite assessments must be completed by these professionals no later than 60 days after the expiration of the PHE. Pennsylvania Senate Democrats
Pennsylvania Senate Democrats  The FDA has posted
The FDA has posted  The Trump administration has released two new documents to support its plan for reopening the country and its economy:
The Trump administration has released two new documents to support its plan for reopening the country and its economy:  The Department of Health has issued
The Department of Health has issued  The Pennsylvania Health Law Center has launched a “
The Pennsylvania Health Law Center has launched a “ The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has shared its
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has shared its